The U.S. Food and Drug Administration has ordered Pfizer and Moderna to update their experimental COVID-19 shot labels to include warnings about the risk of heart inflammation—specifically, myocarditis and pericarditis—in adolescent and young adult males.
The directive, issued on April 17, 2025, expands existing warnings to cover males aged 16 to 25, broadening the previous age ranges of 12 to 17 for Pfizer’s Comirnaty and 18 to 24 for Moderna’s Spikevax.
The FDA cited insurance claims data showing an incidence rate of 8 cases of myocarditis or pericarditis per million doses for the 2023–2024 vaccine formulas.
"The observed risk is higher among men younger than 40 years for BNT162b2 and in men aged 18–24 years for mRNA-1273," the FDA states in its letters to the manufacturers.
The FDA is also requiring Pfizer and Moderna to conduct post-approval studies to assess long-term heart abnormalities, following evidence that such issues can persist months after vaccination.
After urging the public to adhere to the Biden administration's COVID "vaccine" mandate, the Centers for Disease Control and Prevention acknowledged a "likely association" between mRNA vaccines and heart inflammation, particularly in young males after the second dose.
However, CDC officials told an advisory committee in 2025 that acute myocarditis after a COVID-19 vaccine tends to resolve quickly" and is less severe than myocarditis caused by COVID-19 infection.
Pfizer and Moderna were given 30 days to comply with or contest the FDA’s order, though neither company has publicly confirmed compliance or issued a response to the updated labeling requirements.
The updated warnings coincide with broader FDA regulatory changes under the Trump administration, including stricter requirements for approving updated COVID-19 vaccines for healthy adults under 65, potentially limiting access to high-risk groups.
The announcement of the labeling order, first reported by CBS on Wednesday, coincides with a Senate hearing held Wednesday in which lawmakers probed whether federal health officials downplayed myocarditis risks post-vaccination.
Beyond myocarditis, other reported adverse effects of mRNA vaccines include thrombosis with thrombocytopenia syndrome and rare neurological conditions like Guillain-Barré syndrome.
Reports have raised alarms about sudden deaths linked to mRNA vaccines, particularly in double or triple-boosted individuals.
These claims, however, rely on observational data and lack definitive causation, as the FDA and CDC maintain that myocarditis-related deaths are rare and not directly linked to vaccines.
Critics argue that the CDC’s Vaccine Adverse Event Reporting System has made clear for years that the federal agency under the Biden administration brazenly neglected side effects and dangers of the Covid vaccine.
According to VAERS, more people have died after receiving these so-called vaccines than any vaccine in history; 22,231 died after receiving the shots in 2021 and 12,354 in 2022.
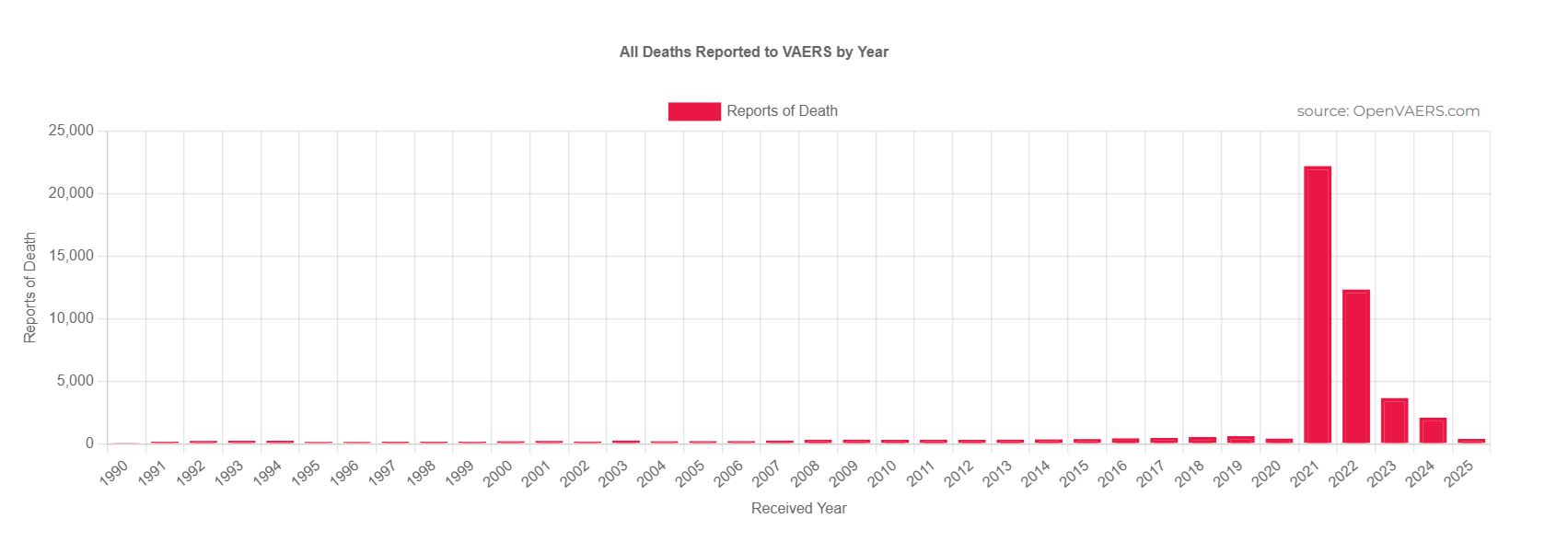
The CDC has reported over 1 million COVID-19 deaths in the U.S. as of May 2025, with COVID-19 ranking as the third leading cause of death in 2021.
Some medical professionals and frontline doctors warn that CDC-directed hospital protocols—including the use of Remdesivir and intubation—may contribute significantly to these deaths, rather than the virus itself.











Conversation